A Couple of Things About the First Law of Thermodynamics
{category_name}So there’s no better way to understand the “laws” of thermodynamics than to actually get a textbook and work some problems! But I’m not going to hold it against you if that doesn’t seem like the best use of your time. Instead then let me attempt to summarize the first and second laws as best I can with an eye to the philosophically salient points required to appreciate their application to life. This post will concern itself with the first law and energy while the following post will discuss the second law and entropy.
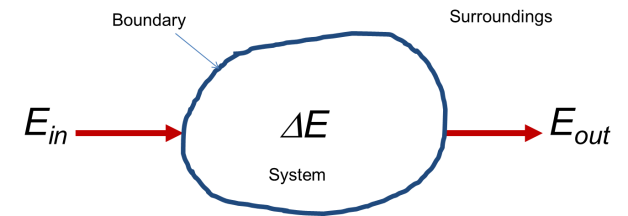
The first law is conservation of energy. It says that the amount of energy in an isolated system cannot change. You may have heard “energy can be neither created nor destroyed.” This is another way of saying that the total quantity of energy in a system cannot change if energy cannot enter or leave from the outside. "System" means any arbitrary portion of the world you care to define, but you have to pick boundaries and you have to know whether radiation or mass can cross those boundaries if you want to be able to calculate things, so you had best to choose your system boundaries carefully! Once this is done though, thermodynamics provides you with a way of predicting what will happen to your system provided you know enough about it. A mathematically convenient way of stating that a quantity is constant is stating that the total change of this quantity is zero, or that ∆E=0. Once you can write that down then you can make an equation that connects conditions in the past to conditions in the future, because you know that the energy will be the same at all times. You’d be amazed at how little we’d be able to predict about nature if this statement wasn't true.
The system, its boundary, and the surroundings are the only objects that exist in the thermodynamic formalism.
Ok so that’s what the first law says but what is energy, anyway? We hear a lot about different kinds of “energies” but the simplest definition of energy comes from the work-energy theorem: Energy is what is capable of doing work. What is work? Work is moving mass over distance, like hauling timber or lifting a coffee cup. If we put all that together then we arrive at the statement: “The total capacity to move mass through space in an isolated system is constant.” I think this statement is an adequate description of the first law. But let me clarify a few points. First, the definition of energy as “how much mass can be moved how far” underscores the fact that energy is a conceptual abstraction. The four forces (but I prefer the word interactions) of nature are understood as the fundamental physical “givens” responsible for this total energy. This is just saying that gravity or electromagnetism, which are the interactions relevant to biology, are the things that “cause” the movement in the mechanistic sense. What we refer to as energy is a way of quantifying the potential for motion that exists because of these interactions. What makes “energy” such a grand unifying abstraction is precisely it’s mathematical property of being a constant quantity. Feynman quipped the following about the first law (my emphasis added):
...It states that there is a certain quantity, which we call energy that does not change in manifold changes which nature undergoes. That is a most abstract idea, because it is a mathematical principle; it says that there is a numerical quantity, which does not change when something happens. It is not a description of a mechanism, or anything concrete; it is just a strange fact that we can calculate some number, and when we finish watching nature go through her tricks and calculate the number again, it is the same.
Two points about this quote: Energy is a numerical quantity. I would restate that point by saying that energy is a mathematical abstraction. It is not “out there in the world” in the way that trees and electromagnetism are “out there.” I’m fond of the assertion that energy is what is, not because energy is a fundamental substratum or essence of “reality” in any sense but rather precisely for the opposite reason that it is a mathematical abstraction which is invariant in time. So that’s the first point--Energy is not a physical thing but a mathematical idea which was “thingified” by language because it was found to have the immensely useful property of never changing no matter how the actual matter under consideration was found to move. The first point is related to the second, which is that the fact of conservation of energy is not a “mechanism.” It is a rule which “mechanics” obeys but it does not tell you how anything happens. So now a brief digression to explain this point.
The discipline of mechanics, whether classical or quantum, is very much in the business of telling us how things happen. When I apply a force to a ball by kicking it I “cause” the ball to accelerate in a mechanistic way, and if someone asks me “how” I made the ball accelerate I can honestly respond “by kicking it.” This is how we would normally render Newton’s second law, f=ma, into language: A force causes a mass to accelerate...so I applied a force with my foot and off the thing went. (Note that it's rather odd to render an equal sign as "cause," but this is how it is generally interpreted at any rate.) Of course, this is not an adequate response to the question of why I kicked the ball and we don’t expect mechanics to answer this question. With this rough distinction between mechanistic how’s and purposive why’s in mind, we come to a platitude repeated in high school courses that I truly despise: Science tells us how things happen, but it requires religion, philosophy, or some form of ethics to discuss why things happen.
I have no idea what does or does not require philosophy, but the assertion about science is only true if we restrict “science” to the disciplines of classical and quantum mechanics. But this is not even all of physics, much less all of science! As I hope you will come to understand, the laws of thermodynamics are about why things happen naturally or, to use the jargon, spontaneously. For the time being suffice it to say that if they explain anything, they explain why a process occurs because they certainly don't explain how anything happens. This is why thermodynamics and mechanics evolved as distinct disciplines until their unification through statistical mechanics (more about this in later posts) and also why mechanics does not deal explicitly with time-directed, irreversible processes.
So “science” has never been in the business of avoiding questions about why things happen and it should not avoid them because its real job is to provide adequate explanations of natural phenomena. Nothing that leaves one with no understanding of why a process occurs could be an adequate explanation. However, biology and the theory of evolution in particular, as I wrote about before, have been particularly damaged by this view that “science” must restrict itself to providing only mechanistic “hows.” In fact, while I’m digressing, in case you haven’t figured it out by now, I don’t believe in Science as such at all. There are only the myriad things people do and measure under more or less controlled conditions in more or less clever or technical ways with concepts that are more or less quantifiable. People who invoke the spirit of Science with a capital S (White lab coats on white males and “pure facts” rendered in the antiseptic UV light of enlightenment reason) most often merely wish to claim blanket authority for ideas which, in the spirit of science, ought to stand on their own.
At any rate that’s what I think you ought to know about the first law. Energy became the central concept of thermodynamics because it has the enormously useful property of being constant in time. How useful? So useful they call it the first law of thermodynamics, and the predictability of the final state of any natural process is grounded in our capacity to write down equations which connect the past and the future. These equations are all in some way derived from the constancy in time of energy. But it is not considered to be the “cause” of anything--gravity and voltages and nuclear forces and so on provide the "how" cause of things. Furthermore, this “law,” or as Feynman says “strange fact,” does not by itself explain why a process does or does not occur. That’s really the second law’s territory, which we’ll get to next time. But understanding entropy, which the second law tells us is the quantity that can only increase, requires this preliminary understanding of energy, the quantity that cannot change. Finally, once we understand the basic ideas of thermo, then we can begin to see how the interaction between that which can’t change and that which can only increase creates these beautiful natural patterns from which we grew.
Fractal pattern indicating self-organization via dissipative flows
Thumbnail image: A forest fire represents a massive transfer of energy from system to surroundings, but the total energy of the universe is unchanged during this process.