A Couple of Things About the First Law of Thermodynamics
{category_name}So there’s no better way to understand the “laws” of thermodynamics than to actually get a textbook and work some problems! But I’m not going to hold it against you if that doesn’t seem like the best use of your time. Instead then let me attempt to summarize the first and second laws as best I can with an eye to the philosophically salient points required to appreciate their application to life. This post will concern itself with the first law and energy while the following post will discuss the second law and entropy.
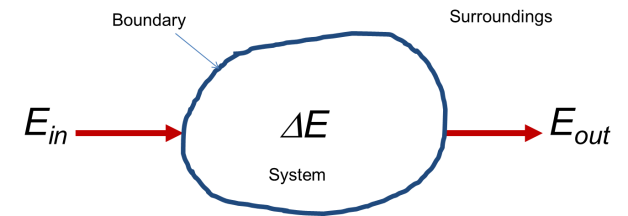
The first law is conservation of energy. It says that the amount of energy in an isolated system cannot change. You may have heard “energy can be neither created nor destroyed.” This is another way of saying that the total quantity of energy in a system cannot change if energy cannot enter or leave from the outside. "System" means any arbitrary portion of the world you care to define, but you have to pick boundaries and you have to know whether radiation or mass can cross those boundaries if you want to be able to calculate things, so you had best to choose your system boundaries carefully! Once this is done though, thermodynamics provides you with a way of predicting what will happen to your system provided you know enough about it. A mathematically convenient way of stating that a quantity is constant is stating that the total change of this quantity is zero, or that ∆E=0. Once you can write that down then you can make an equation that connects conditions in the past to conditions in the future, because you know that the energy will be the same at all times. You’d be amazed at how little we’d be able to predict about nature if this statement wasn't true.
The system, its boundary, and the surroundings are the only objects that exist in the thermodynamic formalism.